In the field of Medical Aesthetics, the currently most commonly used safe dermal filling materials include: synthetic medical silicone, PMMA, polylactic acid (PLLA), hydroxyapatite microsphere and biological collagen, hyaluronic acid, clostridium toxin, autologous fat and other materials. RADIESSE® calcium hydroxyapatite, certified by the US FDA in 2006, is considered as safe and ideal semi-solid injectable facial filler material with good biological properties. The injection site will form new natural tissue under the stimulation of the injectable bioactive material:hydroxyapatite microspheres. The maintenance effect is shorter than the permanent filler PMMA microspheres,but longer than other biological fillers. In terms of safety characteristics, it’s safer than PMMA microspheres, and slightly worse than completely degradable PLLA and biological collagen, hyaluronic acid, autologous fat, etc.
RADIESSE® calcium hydroxyapatite is an opaque, sterile, passive, semi-solid, adhesive implant whose main component is synthetic hydroxyapatite microspheres (particle size range 25-45 μm),which is suspended in a gel carrier containing glycerin , sodium carboxymethyl cellulose, sodium chloride, 0.3% lidocaine hydrochloride and sterile water.
RADIESSE® calcium hydroxyapatite was first approved by the FDA on December 22, 2006. RADIESSE® (lidocaine version) was first approved by the US FDA on January 30, 2015, and was subsequently registered in 57 countries. The product is currently sold around the world, including Europe, Canada, South America and Asia, and reached a sales milestone of 10 million units in 2020. Its market share in the injectable filler market is second only to hyaluronic acid.
In the past nearly 20 years of application history, HAP microspheres have been proven to be an extremely inert and biocompatible raw material. It is not easy to deform during the injection process due to its high hardness. Therefore, it seldomly causes inflammatory stimulation. The way RADIESSE® stimulates the body to produce collagen is purely mechanical conduction. In 21 publications between 2004 and 2015, a total of 5081 Radiesse treatment cases were performed on 2779 patients, and a total of 173 (3%) adverse events (AEs) were reported. Types of AEs assessed included nodules (n=166, 96%), persistent inflammation/swelling (n=4, 2%), persistent erythema (n=2, 1%), and overcorrection (n=1, 1 %).
In 2023, Allergan Aesthetics’ high-end dual-effect hybrid injectable filler:HArmonyCa officially launched the US FDA clinical trial. Currently, HArmonyCa is the only comprehensive filler composed of two active ingredients: hyaluronic acid HA and hydroxyapatite microspheres. It has also been marketed in Europe, Israel and other places.
Facial aging is a multidimensional phenomenon, and its complexity is reflected in changes at multiple anatomical levels not only including the rearrangement of muscles, bones, and adipose tissue, but also the weakening of epidermal elasticity and thickness reduction.
Due to the results of an almost complete study of anatomy, the theory of “facial rejuvenation” focus on “including the remodeling of muscles, bones, fat, and improving the loss of elasticity and thinning of the skin,” while the promotion of new collagen formation is essential to thicken the dermis and improve skin texture and laxity.
Sodium hyaluronate HA is considered as the golden choice for facial rejuvenation treatment, while CaHA calcium hydroxyapatite is the second most commonly used non-surgical cosmetic method. Sodium hyaluronate HA can provide soft tissue volume and may promote collagen synthesis in some cases, while CaHA calcium hydroxyapatite can specifically stimulate the production of new collagen. HArmonyCa is a premixed product that combines highly cross-linked non-animal-derived HA with calcium hydroxyapatite microspheres that has demonstrated positive clinical results in the rejuvenation of the middle and lower face.
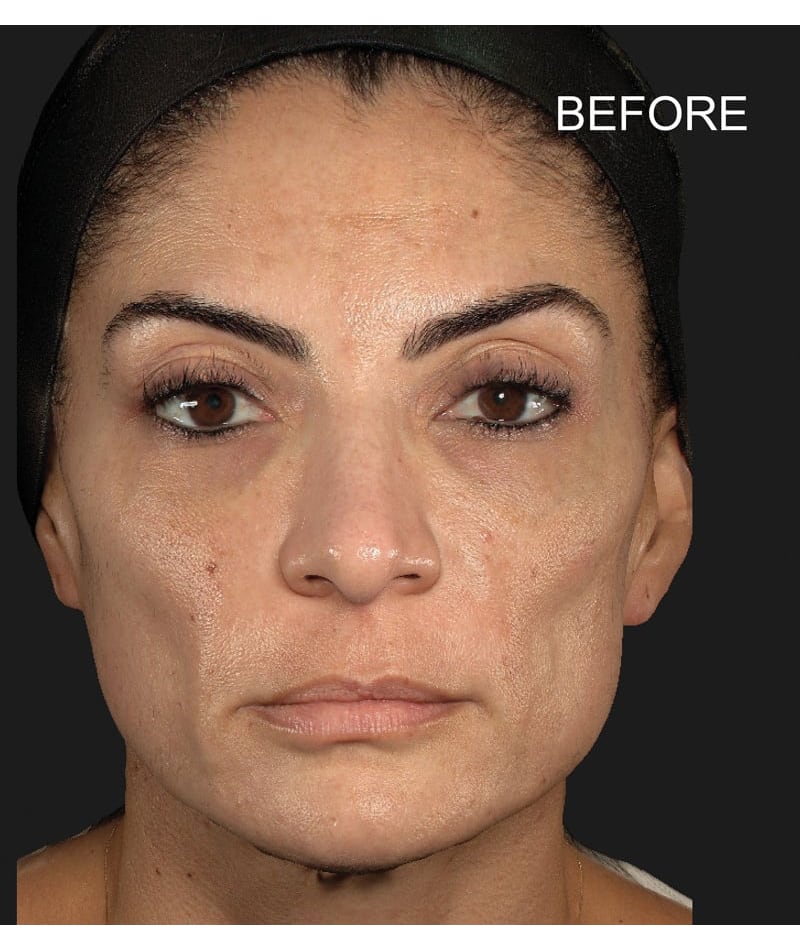
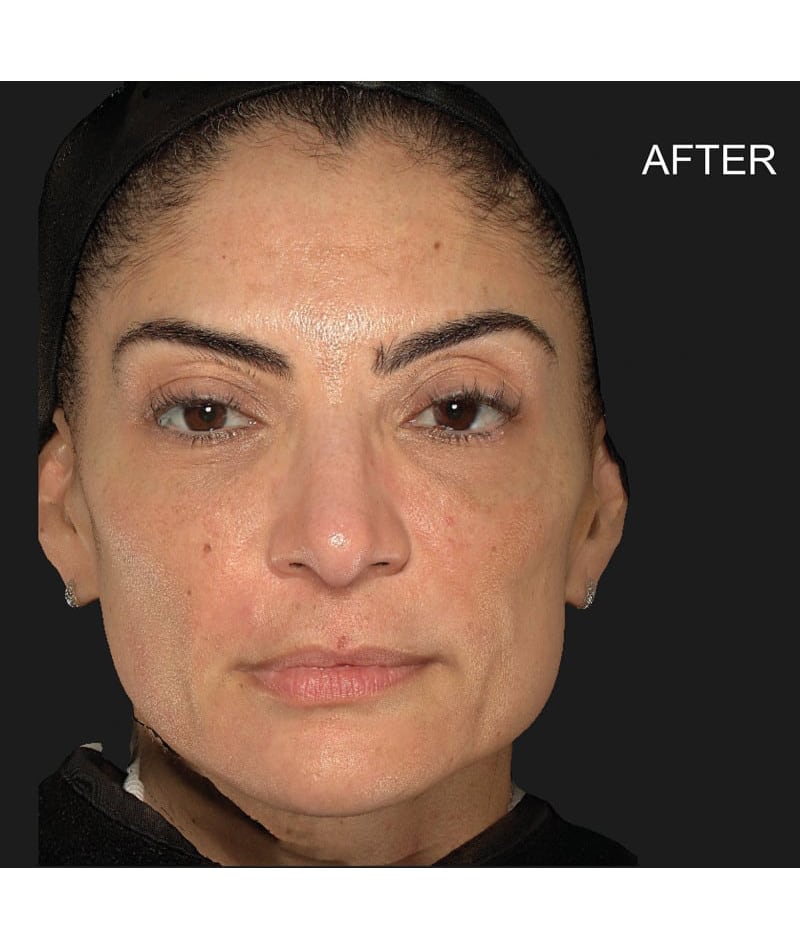
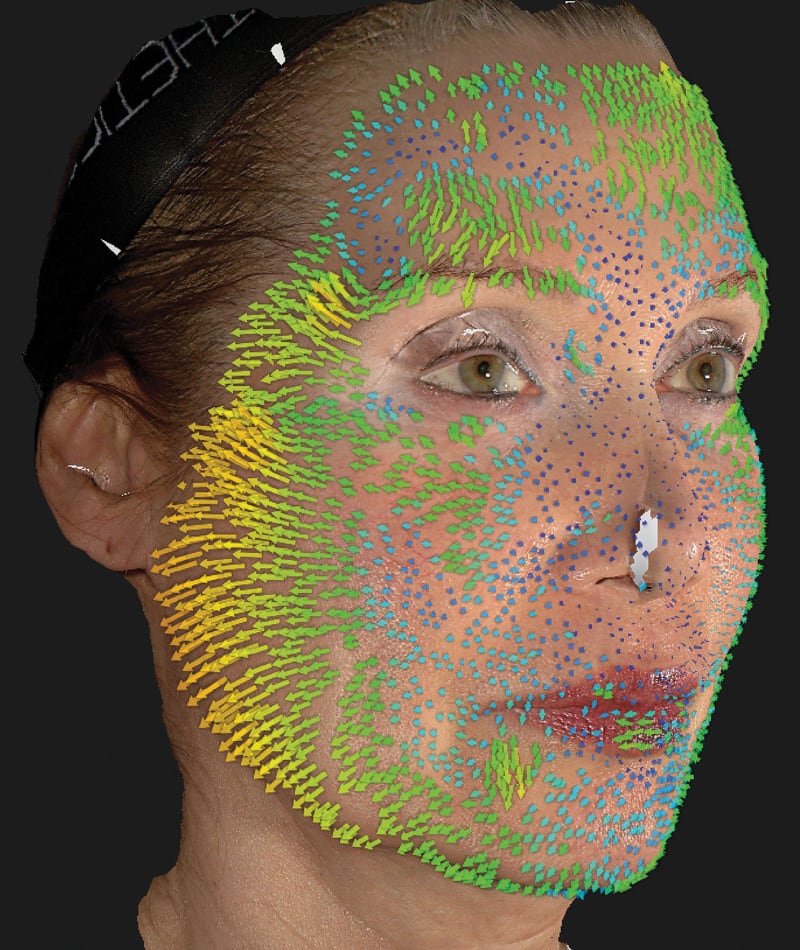
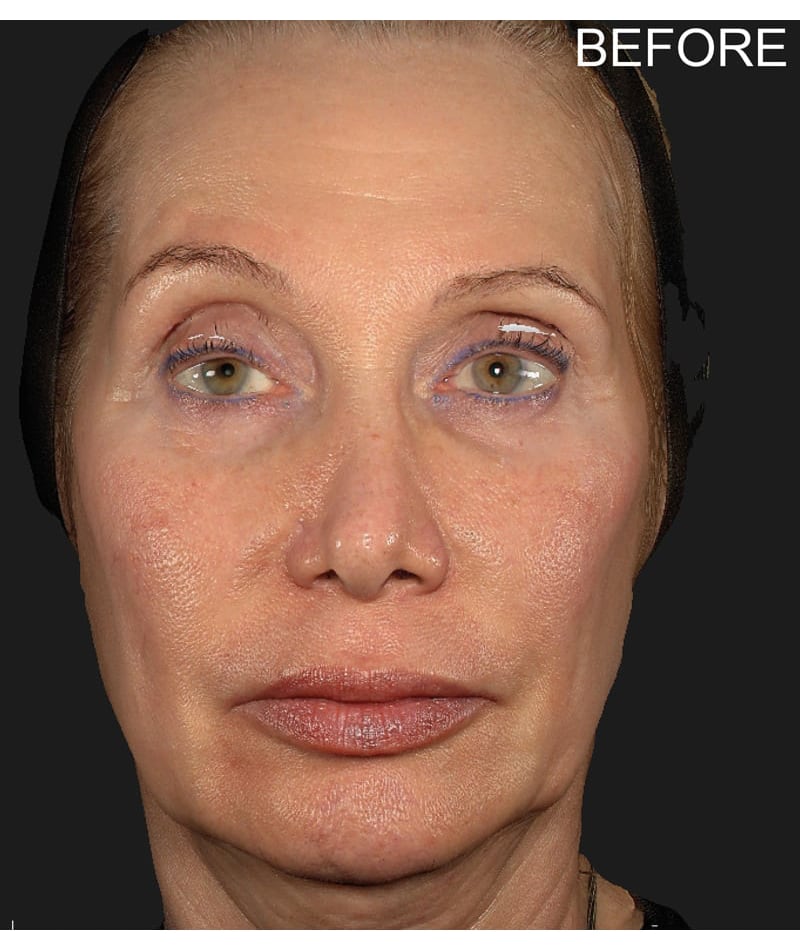
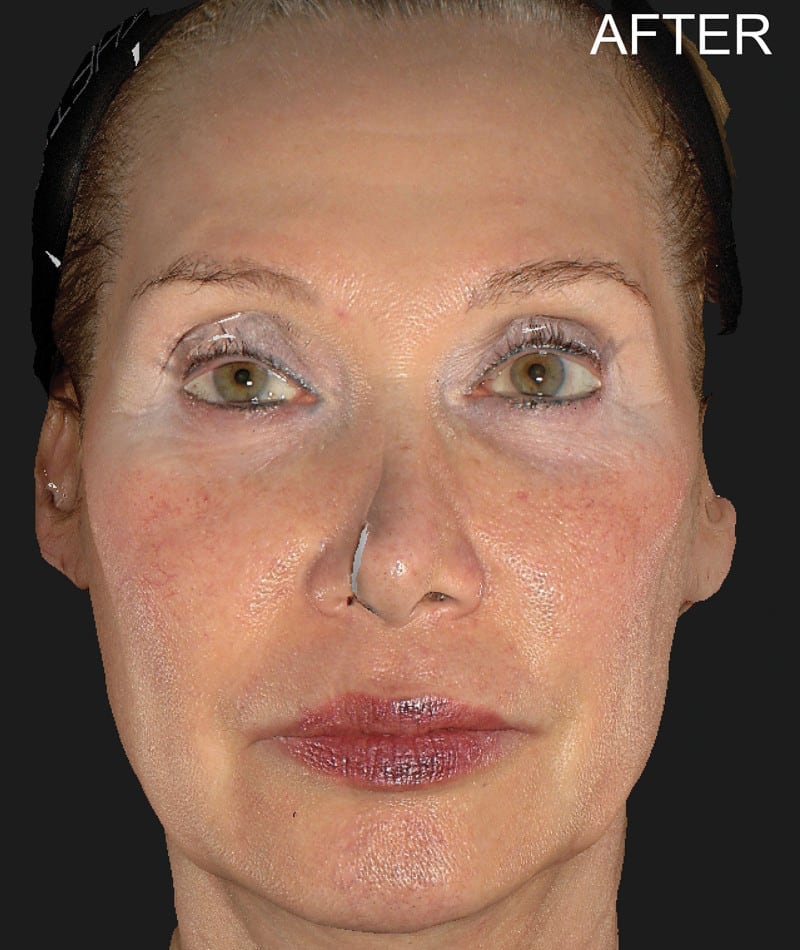
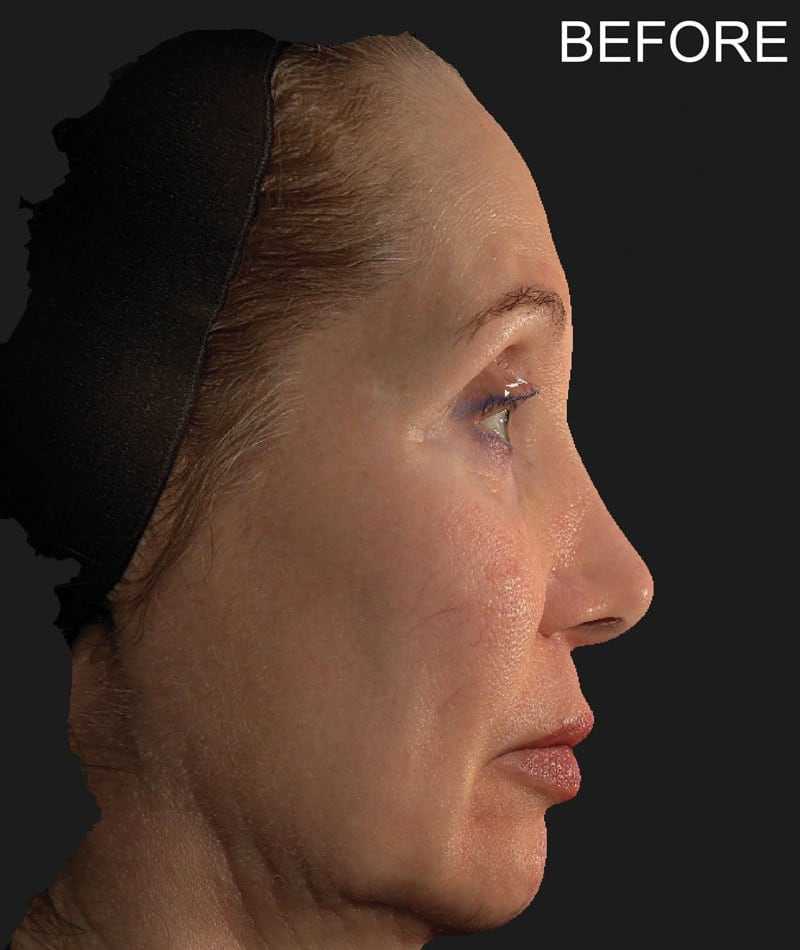
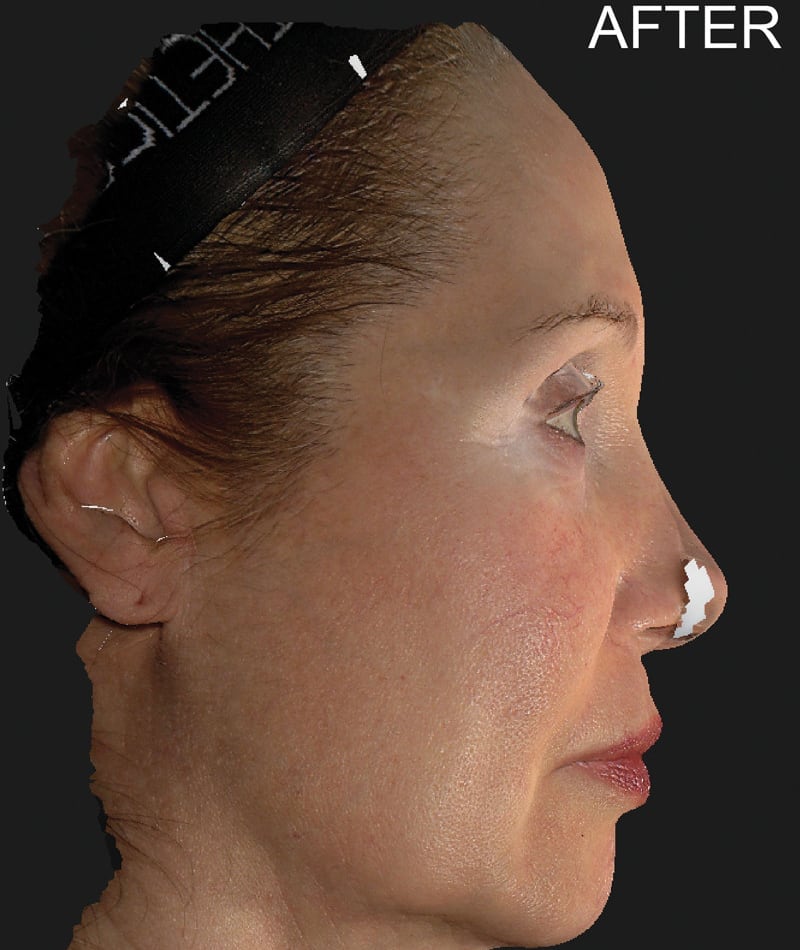
The study used the advanced 3D surface imaging technology Vectra-H2 system to dynamically assess skin surface changes through the label-free tracking function and accurately quantify the degree of skin lifting before and after treatment. The results showed that HArmonyCa™ produced significant tissue lifting effects in the lower face area.
HArmonyCa™, an innovative dermal filler that combines hyaluronic acid and calcium hydroxyapatite microspheres, is not only theoretically expected to bring desirable aesthetic results based on the synergy of the two ingredients, but is also firmly supported through rigorous clinical studies , showing its effectiveness and safety in promoting dermal thickening, improving skin laxity and facial aesthetics. This development provides new treatment strategies for the field of medical aesthetics and is expected to meet the needs of more beauty seekers for natural and lasting facial rejuvenation.