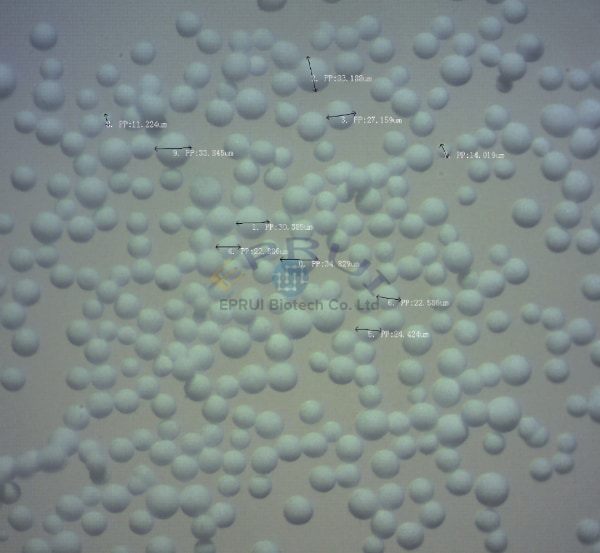
25-45um Calcium Hydroxylapatite Microspheres
Hydroxylapatite is the main inorganic mineral component of animal and human bones. It has good biological activity and compatibility.
Our calcium hydroxylapatite(CaHA) microspheres are 100% synthesized biomaterial similar to the main inorganic components of natural bone.It can form a tight bond with autogenous bone and it is non-toxic, non irritating, non allergenic, non mutagenic, non hemolytic, non destructive to biological tissues.
25-45um smooth calcium hydroxylapatite (CaHA) microspheres provided by EPRUI Biotech has smooth surface can be an affordable substitute to calcium phosphate microspheres provided by Merz Aesthetics.
EPRUI Biotech Trusted By
Facts About Medical Grade CaHA Microspheres
Hydroxylapatite for dermal fillers use is mainly spherical hydroxyapatite calcium (Calcium Hydroxylapatite) with a diameter of 25-45um. It is a kind of biological soft ceramics sintered at high temperature.
By forming a strong chemical bond with bone, making it easy to shape,HAP microspheres are widely used in bone tissue repair and medical aesthetics. Microcrystalline porcelain (Radiesse) ® is a kind of synthetic calcium phosphorus compound in white colloidal form. It is composed of 30% hydroxyapatite microspheres (Calcium Hydroxylapatite) and 70% gel (sodium carboxymethyl cellulose).
The calcium hydroxyapatite biomaterial has been used for decades in dental and orthopedics. Its safety and stability have been verified. After successfully preparing micron-sized spherical hydroxyapatite, HAP microspheres began to be applied in medical aesthetics to help smooth wrinkles.
1. EPRUI CaHA Microspheres Specifications
| Item No. | Morphology | Particle Size | Purity | Application |
|---|---|---|---|---|
| 07-25-45u | Spherical | 25-45um | 99% | HAP Dermal fillers, which is a clone to HAP microspheres used in Radiesse. |
2. What is Radiesse?
Radiesse is calcium hydroxylapatite(CaHA) based dermal filler comprised of 30% CaHA microspheres and 70% aqueous carboxymethyl cellulose(CMC) gel. It was first FDA approved in 2006 for the treatment of human immunodeficiency virus(HIV) associated facial lipoatrophy and moderate to several facial wrinkles, particularly the nasolabial folds. It has since expanded into new formulations and has been approved for jawline contouring and treatment of dorsal hand volume loss.
3. What is the mechanism of action of Radiesse?
Calcium hydroxyapatite microspheres provide the basis of the dermal filler’s appeal. Due to CaHA’s biosimilarity to the hydroxylapatites,it serves as scaffolds for collagen formation in bone matrix and induce biostimulation of stromal components including collagen,elastin and vasculature.
4. Is CaHA dermal filler safe?
Radiesse®, the first CaHA dermal filler which received FDA approval and can be dated from December 22, 2006. On January 30, 2015 , The Radiesse lidocaine version was first approved by the US FDA and subsequently got registered in 57 countries. This product is currently sold worldwide for nearly 20 years, including Europe, Canada, South America, and Asia, and reached a sales milestone of 10 million units in 2020, second only to hyaluronic acid in the injection filling market share.
In the nearly 20-year application history, this calcium hydroxylapatite based dermal filler have been proven to be a highly inert and biocompatible raw material with high hardness, making the CaHA microspheres less prone to deformation during injection and less likely to cause inflammatory stimulation in the body. Therefore, their stimulation of collagen production in the body is purely mechanical conduction. Between 2004 and 2015, 21 literature analyses were conducted on 5081 cases of Radiesse treatment in 2779 patients, reporting a total of 173 adverse events (AEs) (3%). The assessed AE types include nodules (n=166, 96%), persistent inflammation/swelling (n=4.2%), persistent erythema (n=2.1%), and overcorrection (n=1,1%).
5. How long does the effect last after injecting calcium hydroxyapatite(CaHA) based dermal filler?
After injecting CaHA based dermal filler, the result usually lasts for 12 to 18 months.
After injection of Radiesse for 4 weeks, the CMC gel is absorbed, and fibroblasts appear. The process of neocollagenesis begins, stimulating the gradual growth of the patient’s own collagen.
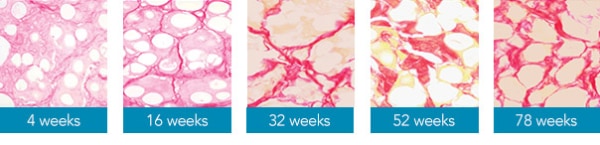
6. What are the potential side effect of injecting CaHA dermal filler?
Potential side effects of injecting CaHA dermal filler include temporary pain,bruising, swelling, redness, itch and discomfort from the injection, allergic reaction, infection, and lumps or nodules, as well as an undesirable cosmetic outcome.
7. Why choose spherical HA from EPRUI?
Accoding to “Degradation of Biomaterials” by Noel L. Davision: Sharp angular shapes can induce a higher response than rounded shapes.
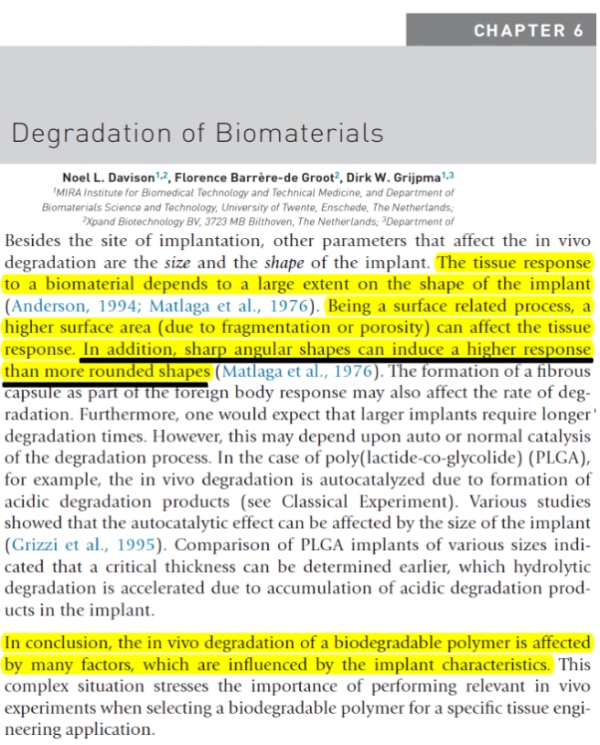
What’s more, our 25-45um calcium hydroxyapatite microspheres have smooth suface,which is a clone to the CaHA microspheres used in Radiesse, but with much more competitive price!
It is used for its characteristic ability to stimulate neocollagenesis in the skin secondary to CaHA’s biosimilarity to the hydroxylapatites which serve as scaffolds for collagen formation in bone matrix.
8. How to order
Please send e-mail to: sales@epruibiotech.com or call us by 86-21-64192663 for products inquires.
For detailed steps, please visit: How to Buy